Minerals and mines
Introduction | History
of lead mining | History
of zinc mining | Lead
ore & mines
Zinc ore & mines | Iron
ore, ochre & mines | Coal mining
Zinc ore and mines
Mineralisation
The lead, zinc and copper veins on Mendip were deposited by hot mineralising fluids (typically between 50 and 150 ° C) rising up from depth and depositing various minerals as they cooled. The source of the fluids were the deep sedimentary basins either side of the Mendips. As the Carboniferous, Triassic and Jurassic sediments in these basins were buried, compacted and heated over time, some of the water in the rock was forced out, along with any dissolved metals and migrated into the neighbouring Carboniferous Limestone. Here the change in chemistry, temperature and pressure led to the deposition of various minerals including lead, zinc, and locally copper.
Types of zinc ore

Zinc ore occurs in two types of deposit: as primary zinc ore in thin veins known as rakes, or a secondary deposit formed by weathering of the primary mineral veins. Zinc ore is most commonly found as zinc carbonate (ZnCO3), known as calamine or smithsonite. It generally occurs as rounded, crystalline crusts or granular, honeycombed masses that have a vitreous or pearly luster and are typically dirty brown or grey in colour. Locally it was known as ‘dry bone’ ore, having a cellular to spongy appearance reminiscent of dry bone. Calamine is actually a secondary mineral, found principally in the oxidized zone of the zinc-bearing ore deposits. It is derived from the alteration of the primary zinc sulphide (ZnS) mineral sphalerite. This is generally a dark grey or black, highly lustrous mineral, but can vary in appearance. Both occur on Mendip.
Its chief use was to make brass by mixing with copper.
Primary zinc veins
The primary zinc ore, sphalerite is typically found in thin veins cutting through the rock. In these veins, the ore occurs as either thin layers encrusting on the walls of the vein, or as thin bands, pockets or crystals within the vein. The veins were always associated with other waste minerals known as ‘gangue’, usually calcite (CaCO3), pyrite (FeS2) or barytes (BaSO4). Many of these veins were very thin, sometimes only a few centimetres wide, and often pinched and swelled along their length, sometimes forming complex anastomosing networks with other veins.
Secondary deposits
In the upper parts of the vein, percolating oxygenated groundwaters cause alteration and breakdown of the primary sulphide minerals. This chemical reaction was enhanced by the presence of pyrite, which when oxidized produces sulphuric acid. The zinc liberated by the oxidation of sphalerite reacted with the carbonate from the host rock to form calamine. This process often destroyed the original vein, instead creating a series of pockets or vugs infilled with calamine.
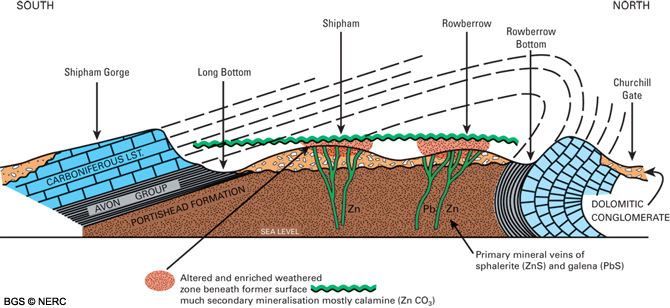
Orefields

Zinc was principally mined around Shipham and Rowberrow, where abundant evidence of the mining activity can be seen in the form of ‘gruffy ground’. This hummocky landscape is formed by the numerous shafts and trial pits excavated by the miners. The principal ore mined around Shipham was calamine, the sphalerite only being of secondary importance. Several old mines are still accessible to cavers. Calamine was also locally mined elsewhere, particularly around Harptree.
The main use of calamine was in the brass industry, where it was mixed with copper to form brass, but it was also sold to pharmacists for its medicinal properties. Today calamine lotion is still available as a soothing and mild antiseptic lotion for use on irritating skin conditions.
- Home
- Overview maps
- Locality
areas
- Cheddar Gorge
- Charterhouse
- Blackdown
- Burrington Combe
- Shipham & Rowberrow
- Crook Peak & Axbridge
- Banwell to Churchill
- Priddy
- Harptree & Smitham Hill
- Draycott & Westbury-sub-Mendip
- Wookey Hole & Ebbor Gorge
- Wells
- Great Elm & Vallis Vale
- Mells & the Wadbury Valley
- The Vobster area
- The Whatley area
- Torr Works & Asham Wood
- Beacon Hill
- Stoke St Michael & Oakhill
- Holwell & Nunney
- Shepton Mallet & Maesbury
- Gurney Slade & Emborough
- The Nettlebridge valley
- Geology
- Minerals and mines
- Quarrying
- Caves and karst
- Biodiversity
- Detailed site information
- Acknowledgements
- External links
- Search
- Site map