How can CO2 be stored?
CO2 can be stored in three main ways:
- in deep geological formations
- in deep ocean water — ocean storage
- in the form of mineral carbonates — mineral storage
Deep ocean storage will increase ocean acidification, a problem that also stems from the excess of carbon dioxide already in the atmosphere and oceans.
Geological formations are currently considered the most promising sequestration sites.
Areas such as the North Sea and the US Gulf Coast are believed to contain a large amount of geological storage space.
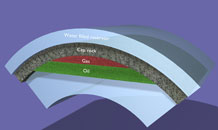
Deep geological formations
Storage in deep geological formations is also known as ‘geo-sequestration’.
In this technique carbon dioxide is converted into a high pressure liquid-like form known as ‘supercritical CO2’. Supercritical CO2 behaves like a runny liquid, rather like WD40.
This supercritical CO2 is injected directly into sedimentary rocks. The rocks may be in old oil fields, gas fields, or in saline formations. Unminable coal seams and saline water-filled basalt volcanic rocks have also been suggested as storage sites.
Various physical, e.g. impermeable ‘caprock’, and geochemical trapping mechanisms prevent CO2 from escaping to the surface.
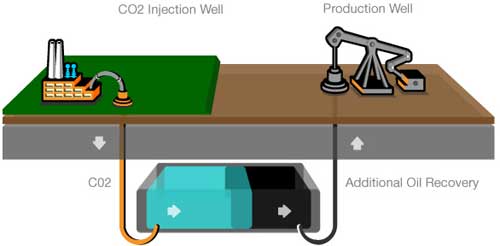
Enhanced Oil Recovery (EOR)
This process is already understood and has been carried out for many years in the process of ‘Enhanced Oil Recovery’ or EOR. In the United States approximately 30 to 50 million tonnes of CO2 are injected annually into declining oil fields. This option is attractive for geological storage because the geological character of hydrocarbon reservoirs is well known, and because costs of injection may be partly offset by the sale of additional oil that is recovered.
The main disadvantage of using old (or ‘depleted’) oil fields is their geographic distribution which may not be ideal in relation to point sources and their limited capacity in comparison with the size of big point sources. Also the subsequent burning of the additional oil so recovered through EOR will offset much or all of the reduction in CO2 emissions.
‘Unminable’ coal
‘Unminable’ coal seams (or coal that is too deep or difficult to mine) can be used to store CO2 because it is adsorbed in the coal if the coal is permeable enough to allow CO2 to penetrate. In the process of absorption the coal releases previously absorbed methane, and the methane can be recovered. This methane can be used and the process of extracting useful methane from a coal seam when it is injected with CO2 is called ECBM (or enhanced coal bed methane).
The sale of the methane can be used to offset a portion of the cost of the CO2 storage. However as in EOR, burning the resultant methane would produce CO2 which would reduce some of the benefit of storing the original CO2.
Saline aquifers

Some deep rock formations contain highly concentrated brine which is present in the rock pores (which act together like a huge sponge), and have so far been considered of no benefit to humans. These are known as ‘saline aquifers’.
In the past they have been rarely used for storage of chemical waste. However the value of saline aquifers is being re-examined.
Their main advantage for CCS is their large potential storage volume and their common occurrence. For example much of the North Sea and mainland Europe and the Texas Gulf Coast is underlain by large saline aquifers.
Their main disadvantage is that relatively little is known about them, compared to oil fields. Unlike storage in oil fields or coal beds no useful by-product will offset the cost of storage.
We need to understand more about saline aquifer storage, but current research shows that several trapping mechanisms immobilise the CO2 underground, reducing the risk of leakage. The IPCC says that for well-selected, designed and managed geological storage sites, CO2 could be trapped for millions of years, retaining over 99 per cent of the injected CO2 over 1000 years.
Ocean storage

Another proposed form of carbon dioxide storage is in the deep oceans, but the environmental effects of this are generally believed to be bad, and the process is not well understood.
Large concentrations of CO2 kill ocean organisms as CO2 acts as an asphyxiant. Also, as CO2 reacts with the water to form carbonic acid, H2CO3, the acidity of ocean water would increase.
Mineral storage

In mineral storage, captured CO2 is reacted with naturally occurring magnesium- (Mg) and calcium- (Ca) containing minerals. This is called mineral carbonation and occurs naturally as the weathering of rock over geologic time periods.
Such magnesium and calcium minerals are very abundant and are very stable. As a result, the re-release of CO2 into the atmosphere does not happen.
However, these carbonation reactions are very slow under normal temperatures and pressures and to speed it up would need energy. The reaction rate can be made faster, by reacting at higher temperatures and pressures, or by pre-treatment of the minerals.
The IPCC estimates that a power plant equipped with CCS using mineral storage will need 60–180 per cent more energy than a power plant without CCS.